Why I Invented Happy Atoms
By Jesse Schell
Happy Atoms is a project that has been germinating in my brain for about forty years.
It all began in the library of Riverview Elementary School, where I stumbled upon the Scott Corbett "Trick" books. This was a series of children’s books about a boy named Kerby Maxwell who becomes the owner of a magic chemistry set that becomes the center of his many adventures.


These books seem to have gone out of fashion today, perhaps because the idea of a boy who lives by the motto "A dedicated scientist never hesitates to experiment on himself," and mixes and drinks unlabeled chemicals given to him by a stranger might not seem completely safe to today’s parents. But to me, it was the stuff of inspiration. Despite my pleas, however, my parents were not ready to invest in a chemistry set, which left my alchemical tinkerings in the realm of vinegar and baking soda.
But chemistry stayed on my mind. As I grew older, I was fascinated to learn about the periodic table — the notion that everything in the universe was made up of a patterned set of one hundred microscopic building blocks seemed absolutely amazing. In school we learned that water was H2O, and salt was NaCl, but I wanted to know so much more! I beleaguered my poor science teacher with questions about chemical formulas — what’s the formula for paper? For glass? For air? For rocks? For blood? For gasoline? For plastic? Can every atom combine with every other atom? The answers were dispiriting. I was usually told either "it’s too complicated," or "go look it up," which of course is adult speak for "I don’t know." I wanted to look these things up… but where? How? If everything in the universe can be represented by simple formulas, why aren’t they in my science book? Why am I forced to memorize the symbols of the periodic table if we aren’t going to use them for anything?

Molecules and Happiness
Eventually I got to high school, and was very excited to get into an honors chemistry class. I learned a lot of interesting things there about electron orbitals, measuring pH, how to balance chemical equations, and of course lots and lots of memorizing the periodic table, but something was missing. We learned about how many neutrons each element has, and what happens when you mix acids and bases, but we learned almost nothing about how molecules were formed. I could tell you how many protons were in beryllium, but where was beryllium in the world? I felt as if I’d learned the letters of the alphabet, but never learned how those letters formed into words.
In college I studied computer science, which required more chemistry classes, and at last, eureka! We started to learn something about how atoms formed into molecules! I was most fascinated with Lewis diagrams, which are a way of showing how atoms share electrons. It’s a simple but strange notion — each atom innately has a certain number of electrons in its outermost shell, but wishes it had more. Hydrogen, for example, has one electron, but it wishes it had two. As a result, hydrogen atoms tend to pair up, sharing their electrons so that they each have access to two. Oxygen has six, but wishes it had eight. If it meets up with two lone hydrogens, it can make them happy by sharing an electron with each of them, and they can make it happy by sharing their electrons with oxygen. Of course, it’s silly to think of atoms being happy or sad — all we are talking about is the fact that atomic forces make atoms stick together in certain ways — but as humans, we tend to anthropomorphize everything, because it is easier to think about, and even professional chemists talk about what different atoms “prefer.”
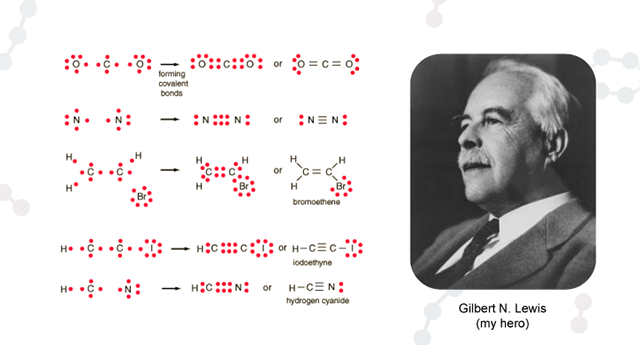
The Diagram Dilemma
I was in love with this notion of using simple diagrams to figure out which atoms would combine with which others, and the margins of my notebooks started to fill with Lewis diagram doodles of molecules. But soon, new frustrations arose: I could draw diagrams of all kinds of interesting looking molecules. I could easily see that C2H6O was likely to be a stable molecule… but what was it? I wanted to develop intuition about how molecular structure made things behave in the real world — but how could I do that if I couldn’t even figure out what the formulas represented?
While this troubled me, it was no trouble at all in terms of my classes. An intuitive grasp of how chemical formulas relate to the real world was not something that was needed to pass — I found all that was necessary was to learn how manipulate various abstract formulas, which as a computer science student, was not hard to learn. I never much understood what any of it really meant, but no one seemed to mind that but me.
And that could have been the end of my chemistry career. I moved on to become a professional software engineer, and eventually a computer game designer. But I never forgot the Lewis diagrams that intrigued me so much, and would occasionally find myself doodling out molecules, and wondering what they really were, and if they really existed. Years later, when I was teaching at Carnegie Mellon, I found myself wondering if creating a digital representation of Lewis diagrams could be the foundation of a computer game. I sketched out some ideas, and worked with a graduate student, John Kolencheryl, to build a prototype. It was a crude game that involved dragging electrons around to try to make all the atoms in the vicinity “happy.” It had a lot of flaws, but it did work. Only one thing troubled me — playing it felt very abstract. Manipulating the electrons was tedious. I found myself wishing I could just reach my hands into the game, grab the atoms, and bring them together like the physical matter they were.
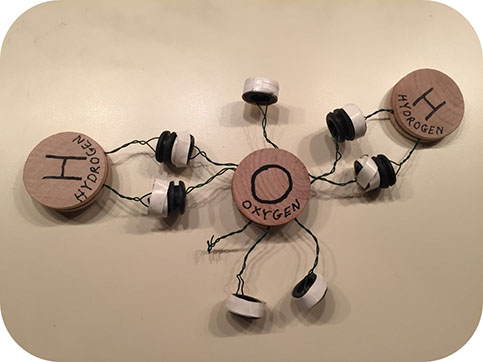
Then one day I got an email from our local elementary school. They were having a “science decathlon” — would I mind coming in to talk about how computer games are made? On a whim, I wrote back saying that I could do that, but I could also give a demonstration about how atoms formed into molecules — would that be okay? The teacher thought that sounded even better, so suddenly I was on the schedule. Now I had to think — how was I going to do this? I started some experiments with cloth and ribbons and Velcro to make models of how atoms used electrons to connect, but the Velcro was hard to work with. I needed something more solid. I tried some experiments with snaps, and they worked great! In a crafting frenzy, I made dozens of atoms out of wooden discs, colored ribbons, metal snaps, and lots and lots of hot glue.

The Building Blocks of the Universe
On the day of the “science decathlon,” I donned a lab coat I bought on the Internet and nervously faced a roomful of antsy fifth and sixth graders. I asked them to think about what would happen if you kept dividing a drop of water in half — could you do it forever? No, they said with certainty, eventually it would be too small to see. “But I have a super microscope,” I countered, “I can see the drop no matter how small it gets.” They looked at each other uncertainly. They had no idea. I asked who knew what an atom was. A small number of hands. I started to panic — how was I going to explain electron bonding to students who have never even heard of an atom? I calmly explained that you can only divide a drop of water in half about seventy five times before you can’t go any farther — that there is a smallest drop of water that can’t be broken up any further, and it is called a “molecule.” Brows furrowed as they stared at me with some suspicion. Then I asked if anyone knew the formula for water — and every hand in the room shot up! “H2O!” they shouted excitedly. I reeled for a moment at the realization that their education had provided them with this fact, but had not yet managed to connect it to any real meaning. “And what does that mean — does anyone know?” Many hands went down. One student nervously ventured “One hydrogen and two oxygens?” “Very close! Two hydrogens, and one oxygen!” I then apologized for chemistry’s strange policy of putting the adjective after the noun. “Every bit of water in the world is made of these building blocks — two hydrogens, and one oxygen. We call these building blocks ‘elements,’ And everything in the entire universe is made up of building blocks like this — just like Legos. How many types of blocks do you think there are?” They looked at me incredulously. “In the whole universe? Must be zillions!” called one. “Nope,” I replied. “There are only about one hundred. And this,” I pointed to the periodic table, “is the list of all of them. Everything in the entire universe is made of these one hundred or so Lego blocks, and nothing else. And most of the things you see only use about thirty of them. Today, I’m going to show you how to join them together to make everything in the universe.”
I gave each student one atom, and explained that most atoms, when they are alone, are unhappy, because they have unconnected spots. You make them happy by connecting them together into molecules. I told them I’d give out points for each of the molecules they could make — one point for each atom in each molecule. Thrilled to be able to get out of their seats, they started forming into groups and snapping together my primitive models, then bringing them for me to inspect. Some were malformed, with simple mistakes which I could correct, but quickly students started bringing me small, properly formed molecules. Two boys brought a tiny molecule, and I explained “Two hydrogens together! That’s hydrogen gas! It used to be what they put into blimps — but they stopped because it kept exploding.” “BOOM!” shouted the boys gleefully, and ran off to see what else they could make. Two girls brought a pair of oxygen atoms. “Does this work?” they asked uncertainly. “Not only does it work — this is the oxygen molecule! It’s in the room all around us — if we go more than five minutes without breathing it, we die.” Their eyes got big, and they went off to make more. Gradually, the students started making more and larger molecules that were beyond my ability to recognize. They seemed to like stumping me just as much as learning what they had made. Gradually, the student groups got larger and larger, as they set to form the largest molecules they could manage. Like doctors around an operating table, they all studied what they were making, and frantically tried to connect things together properly. I was astonished to hear them calling out “Two open spots here! Who has an oxygen? Wait… sulfur would also work!” or “I either need a nitrogen, or three hydrogens — can anyone help?”
After thirty minutes of enthusiastic play, I called time. They returned to their seats, somewhat exhausted, but excited to see the dozens of molecular formulas on the blackboard that they had created together. I asked them, “When we started, did you think you could make this many?” “No way!” They said. “I guess we’re pretty good at this!” “I guess you are!” I agreed. Then I asked them what they learned about the different atoms they had been playing with. Lots of hands went up. “Hydrogen is little, but it’s really useful,” said one student. “There are lots of kinds of salts,” said another. “Carbon connects to everything,” said a third. “Were there any atoms you didn’t like?” I asked. “Yeah, beryllium,” said a frowning boy, “I got stuck with that, and it has a really hard time connecting with things.” “It’s not as bad as helium and neon,” countered a girl. “They are already happy, and never connect with anything, ever!” Then the bell rang, and they thanked me, and filed out, leaving me with their teacher, whose mouth was hanging open. “I have never seen anything like that in my life!” She exclaimed. “When you started, I was sure this was going to be over their heads — but they got it so fast! And they were so excited!” I told her I was as surprised as she was — I had no idea they would understand it so easily, or that they would figure out so much on their own. All I had done was give them five minutes of explanation about what atoms were, and how they connected, and everything else they figured out on their own. I ran the demonstration with a few more classes, and got the same results each time. By the end of the day I was certain that I had something that had potential.
Building on the Potential
As I reflected on it over the coming weeks, I found myself focusing more on the flaws of my models. The snaps were a little hard to use. The ribbons tended to get tangled. And most importantly, the students kept making molecules I couldn’t identify. I made a new set that used magnets and wires instead of ribbons and snaps — and it was a big improvement. It was really fun to fiddle with, and it was really cool how the magnets would pull the atoms together, in a rough simulation of how they really connect. When I would make a triple bond between two nitrogen atoms, I could really feel the pull of the forces holding them together. But how to solve the problem of identifying the molecules? So many different molecules were possible — what I really needed was a computer to do the identification. Thinking about many solutions, I kept coming back to the idea of a computer vision algorithm.
Every year, at Schell Games, we do a thing called “Jam Week,” where we stop all our normal projects, and work on nothing but passion projects for one week. I described the problem I was trying to solve to several people at the studio, and Russell Ball stepped up and offered to give it a try. I spent the week making atoms out of colored spheres and magnets, and Russ experimented with different vision algorithms, trying to recognize and identify colored spheres in digital photographs. Results were really promising! The algorithms needed to be tricky, but some basic identification was definitely possible.
Not long after, Steve Schneider from WestEd approached me: the US Department of Education was giving out grants to small businesses that had good ideas for teaching science — did Schell Games have any projects that might fit? We’d never tried applying for grants before — but this did seem like a good fit, so we applied. To our astonishment, it got funded! Suddenly, my basement hobby project was a real, funded project we were on the hook to create and distribute! We were excited… but also terrified!
Step by tentative step, we found our way forward. Chris Patlovany, an intern, did all kinds of experiments with the right way to make our physical models. Yotam Haimberg, one of our game designers, happened to have a degree in chemistry and was an ideal director for the project. We found a phenomenal subject matter expert, Dr. Catalina Achim, whose specialty was how to best teach chemistry to younger students. Mike Lee took over vision algorithms and started to have great success. We hired Brooke Morrill as a full-time educational researcher, who helped so much coordinating and writing the much more complicated phase II grant proposal… which we received! That night, we were celebrating with a Chinese dinner, and my fortune cookie read “An old wish will come true.” I wondered what it meant at first — and then suddenly I thought back to the third grade, when I first discovered the joy of invention, and was determined that one day I would grow up to be an inventor. I used to dream that one day, someone would say to me “Your invention is so great that I’m going to give you a million dollars.” And now that had actually happened!

Evolution
Phase II funding gave us a lot more confidence and ability to get the project finished. We put together a full team, whose first step was to create the design pillars that the whole project was formed around.


Cool new ideas started to rise up, like representing the world of molecules as a map that players could gradually discover, like explorers of a new world. Then, as we looked around for partners who could help with manufacturing and distribution, we met up with Thames and Kosmos, one of the largest science kit manufacturers in the world. They were interested and enthusiastic, and we decided to team up together. We showed an early prototype at 2016 Toy Fair at their booth, and we were excited to win a “Best of Toy Fair” award!

I worry that I’m making it sound like the development of Happy Atoms was obvious, and an easy road. In reality, it has been difficult decisions and uncomfortable compromises every step of the way. Not everything that subject matter experts, students, or teachers have had to say has been encouraging. Our simple system of modelling chemical bonds with the octet rule makes certain molecules off limits for our model. Boron and aluminum are conspicuously absent from our periodic table for this reason. Elements with atomic numbers larger than 20 are also a problem for this model. And these concerns are just the beginning — there are many, many ways that the Happy Atoms system is not a perfect model of reality. For a while, this concerned us greatly, and we started to think that we might need to abandon the project; after all, isn’t promoting an imperfect model educationally irresponsible? But we took great inspiration from the chemist Henry Bent, who insists that the purpose of a model is not be accurate, the purpose of a model is to give insight. Or, in the words of philosopher Hans Vaihinger, “It must be remembered that the object of the world of ideas as a whole is not the portrayal of reality — that would be an utterly impossible task — but rather to provide us with an instrument for finding our way about in this world more easily.” And we were finding that our system, though not perfect, was really helping people find their way through world of chemistry.
Helping Everyone Discover the World of Molecules
As we move toward a launched product we are trying hard not to forget the purpose of Happy Atoms: To create an experience where anyone can intuitively understand the world of molecules, just by playing. Understanding chemistry is the key to solving the world’s most challenging problems: curing cancer, purifying our water, finding new energy sources, stopping global warming — all these, and thousands more will be solved by those who understand chemistry. Because of this, I truly believe this is the most important project I have ever worked on. If this project can increase the number of students passionate about entering chemistry-related careers by even a tiny fraction, it could make a tremendous difference in the world. We continue to take inspiration from the story of ten-year-old Clara Lazen, who, with guidance from her science teacher, was given the opportunity to play with a college-level set of molecular models. She built a structure that seemed natural and intuitive to her, and asked her teacher what it was. He didn’t know, and made some inquiries. The chemistry professors he talked to agreed that the molecule she had created should exist — but it was not in any known catalogue. No one had thought to create it before. As a result, Clara and her teacher published a paper together about the structure of tetranitratoxycarbon.
Clara’s story is inspiring, because it shows that one inquisitive child can make a difference in the world of science. Our goal is nothing less than to open the doors to this same experience of playful exploration to millions of children and adults — to create a world where being ignorant about what we are made of is the exception, not the norm; to create a world where anyone willing to play will understand what our world is made of, and how and why it works.


